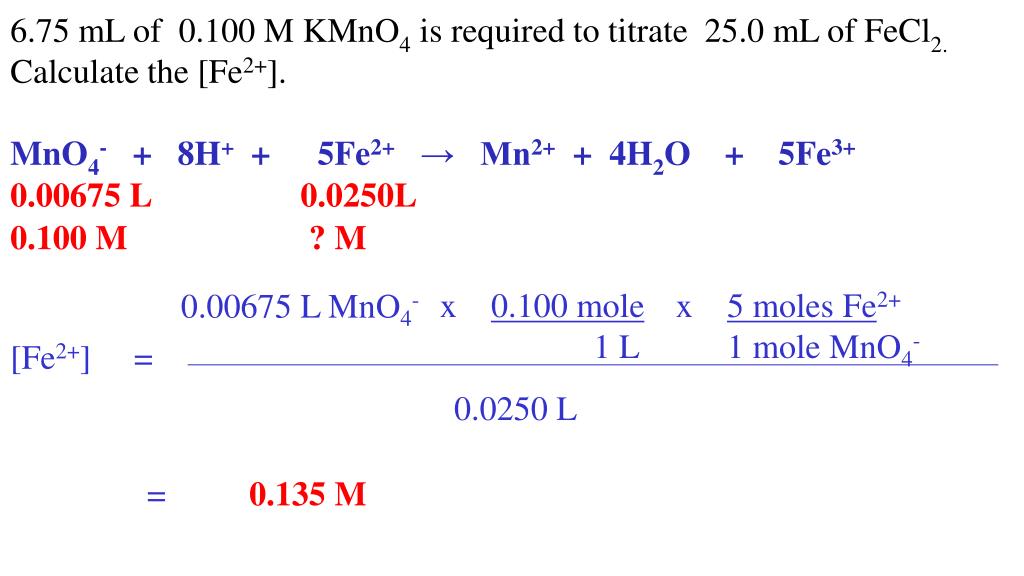
Fe2+ And Kmno4 Equation . 2fe + 2kmno4 = fe2o3 + 2mno2 + k2o is a redox reaction where fe. kmno4 (potassium permanganate) summary of redox reaction. F e2+ → f e3+ +e− (i) and permanganate ion is reduced: in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. it takes five moles of fe 2+ to react with one mole of kmno 4 according to the balanced chemical equation for the reaction. Two moles of iron [fe] and two moles. M no− 4 + 8h. Iron + potassium permanganate = hematite + manganese dioxide + potassium oxide.
from www.slideserve.com
2fe + 2kmno4 = fe2o3 + 2mno2 + k2o is a redox reaction where fe. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Two moles of iron [fe] and two moles. M no− 4 + 8h. F e2+ → f e3+ +e− (i) and permanganate ion is reduced: it takes five moles of fe 2+ to react with one mole of kmno 4 according to the balanced chemical equation for the reaction. Iron + potassium permanganate = hematite + manganese dioxide + potassium oxide. kmno4 (potassium permanganate) summary of redox reaction.
PPT The Breathalyzer PowerPoint Presentation, free download ID5480133
Fe2+ And Kmno4 Equation in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. 2fe + 2kmno4 = fe2o3 + 2mno2 + k2o is a redox reaction where fe. M no− 4 + 8h. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. it takes five moles of fe 2+ to react with one mole of kmno 4 according to the balanced chemical equation for the reaction. F e2+ → f e3+ +e− (i) and permanganate ion is reduced: Iron + potassium permanganate = hematite + manganese dioxide + potassium oxide. kmno4 (potassium permanganate) summary of redox reaction. Two moles of iron [fe] and two moles.
From www.numerade.com
SOLVED In the titration of ammonium iron (II) sulfate hexahydrate with a standard solution of Fe2+ And Kmno4 Equation F e2+ → f e3+ +e− (i) and permanganate ion is reduced: 2fe + 2kmno4 = fe2o3 + 2mno2 + k2o is a redox reaction where fe. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Two moles of iron [fe] and. Fe2+ And Kmno4 Equation.
From www.meritnation.com
What happens when acidified KMnO4 is reacted with (a) Fe2+(b) I (c) S2 (d) C2O4 2 (e)NO2 Fe2+ And Kmno4 Equation Two moles of iron [fe] and two moles. 2fe + 2kmno4 = fe2o3 + 2mno2 + k2o is a redox reaction where fe. Iron + potassium permanganate = hematite + manganese dioxide + potassium oxide. kmno4 (potassium permanganate) summary of redox reaction. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine. Fe2+ And Kmno4 Equation.
From www.numerade.com
SOLVED Write the Redox titration report for determination of Fe2+ concentration by Potassium Fe2+ And Kmno4 Equation Iron + potassium permanganate = hematite + manganese dioxide + potassium oxide. 2fe + 2kmno4 = fe2o3 + 2mno2 + k2o is a redox reaction where fe. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Two moles of iron [fe] and. Fe2+ And Kmno4 Equation.
From www.numerade.com
SOLVED Write the Redox titration report for determination of Fe2+ concentration by Potassium Fe2+ And Kmno4 Equation 2fe + 2kmno4 = fe2o3 + 2mno2 + k2o is a redox reaction where fe. Iron + potassium permanganate = hematite + manganese dioxide + potassium oxide. kmno4 (potassium permanganate) summary of redox reaction. F e2+ → f e3+ +e− (i) and permanganate ion is reduced: M no− 4 + 8h. it takes five moles of fe 2+. Fe2+ And Kmno4 Equation.
From www.slideserve.com
PPT The Breathalyzer PowerPoint Presentation, free download ID5480133 Fe2+ And Kmno4 Equation Iron + potassium permanganate = hematite + manganese dioxide + potassium oxide. kmno4 (potassium permanganate) summary of redox reaction. 2fe + 2kmno4 = fe2o3 + 2mno2 + k2o is a redox reaction where fe. Two moles of iron [fe] and two moles. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine. Fe2+ And Kmno4 Equation.
From byjus.com
Mohr Salt Titration with KMnO4 CBSE Chemistry Practicals Class 12 Fe2+ And Kmno4 Equation F e2+ → f e3+ +e− (i) and permanganate ion is reduced: Two moles of iron [fe] and two moles. M no− 4 + 8h. Iron + potassium permanganate = hematite + manganese dioxide + potassium oxide. kmno4 (potassium permanganate) summary of redox reaction. 2fe + 2kmno4 = fe2o3 + 2mno2 + k2o is a redox reaction where fe.. Fe2+ And Kmno4 Equation.
From www.numerade.com
SOLVED A quantity of 25.0 mL of a solution containing both Fe2+ and Fe3+ ions is titrated with Fe2+ And Kmno4 Equation Two moles of iron [fe] and two moles. F e2+ → f e3+ +e− (i) and permanganate ion is reduced: kmno4 (potassium permanganate) summary of redox reaction. it takes five moles of fe 2+ to react with one mole of kmno 4 according to the balanced chemical equation for the reaction. 2fe + 2kmno4 = fe2o3 + 2mno2. Fe2+ And Kmno4 Equation.
From www.coursehero.com
[Solved] What is the balanced equation between KMnO4 and As2O3. (For Redox... Course Hero Fe2+ And Kmno4 Equation F e2+ → f e3+ +e− (i) and permanganate ion is reduced: it takes five moles of fe 2+ to react with one mole of kmno 4 according to the balanced chemical equation for the reaction. 2fe + 2kmno4 = fe2o3 + 2mno2 + k2o is a redox reaction where fe. kmno4 (potassium permanganate) summary of redox reaction.. Fe2+ And Kmno4 Equation.
From www.youtube.com
FeC2O4+KMnO4+H2SO4 GIVES Fe2(SO4)3+MnSO4+ K2S04+H2O+CO2. Balancing Redox reaction YouTube Fe2+ And Kmno4 Equation it takes five moles of fe 2+ to react with one mole of kmno 4 according to the balanced chemical equation for the reaction. M no− 4 + 8h. kmno4 (potassium permanganate) summary of redox reaction. 2fe + 2kmno4 = fe2o3 + 2mno2 + k2o is a redox reaction where fe. in this experiment you will use. Fe2+ And Kmno4 Equation.
From www.numerade.com
SOLVED Using the halfredox method, what is the balanced redox equation of a. KMnO4 and Na2C2O4 Fe2+ And Kmno4 Equation it takes five moles of fe 2+ to react with one mole of kmno 4 according to the balanced chemical equation for the reaction. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. 2fe + 2kmno4 = fe2o3 + 2mno2 +. Fe2+ And Kmno4 Equation.
From brainly.lat
FeSO4 KMnO4 H2SO4 Fe2(SO4)3 K2SO4 MnSO4 H2O balancear la ecuacion por el metodo de oxido Fe2+ And Kmno4 Equation M no− 4 + 8h. kmno4 (potassium permanganate) summary of redox reaction. F e2+ → f e3+ +e− (i) and permanganate ion is reduced: Iron + potassium permanganate = hematite + manganese dioxide + potassium oxide. 2fe + 2kmno4 = fe2o3 + 2mno2 + k2o is a redox reaction where fe. in this experiment you will use a. Fe2+ And Kmno4 Equation.
From www.youtube.com
General Chemistry Standardization of KMnO4 By Redox Titration YouTube Fe2+ And Kmno4 Equation Two moles of iron [fe] and two moles. kmno4 (potassium permanganate) summary of redox reaction. M no− 4 + 8h. F e2+ → f e3+ +e− (i) and permanganate ion is reduced: in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution.. Fe2+ And Kmno4 Equation.
From www.youtube.com
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions) YouTube Fe2+ And Kmno4 Equation 2fe + 2kmno4 = fe2o3 + 2mno2 + k2o is a redox reaction where fe. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. it takes five moles of fe 2+ to react with one mole of kmno 4 according to. Fe2+ And Kmno4 Equation.
From www.youtube.com
Equation for KMnO4 + H2O (Potassium Permanganate + Water) YouTube Fe2+ And Kmno4 Equation 2fe + 2kmno4 = fe2o3 + 2mno2 + k2o is a redox reaction where fe. Two moles of iron [fe] and two moles. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. F e2+ → f e3+ +e− (i) and permanganate ion. Fe2+ And Kmno4 Equation.
From www.coursehero.com
[Solved] What is the balanced equation between KMnO4 and FeCl2. (For Redox... Course Hero Fe2+ And Kmno4 Equation Iron + potassium permanganate = hematite + manganese dioxide + potassium oxide. M no− 4 + 8h. it takes five moles of fe 2+ to react with one mole of kmno 4 according to the balanced chemical equation for the reaction. Two moles of iron [fe] and two moles. in this experiment you will use a standard solution. Fe2+ And Kmno4 Equation.
From www.numerade.com
SOLVED In the titration of ammonium iron (II) sulfate hexahydrate with a standard solution of Fe2+ And Kmno4 Equation M no− 4 + 8h. kmno4 (potassium permanganate) summary of redox reaction. 2fe + 2kmno4 = fe2o3 + 2mno2 + k2o is a redox reaction where fe. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. F e2+ → f e3+. Fe2+ And Kmno4 Equation.
From www.numerade.com
4The percentage of iron(II) ions, Fe2+, in a vitamin tablet can be estimated by dissolving the Fe2+ And Kmno4 Equation 2fe + 2kmno4 = fe2o3 + 2mno2 + k2o is a redox reaction where fe. Iron + potassium permanganate = hematite + manganese dioxide + potassium oxide. F e2+ → f e3+ +e− (i) and permanganate ion is reduced: it takes five moles of fe 2+ to react with one mole of kmno 4 according to the balanced chemical. Fe2+ And Kmno4 Equation.
From www.youtube.com
How to Balance KMnO4 + Fe = FeO + K2O + MnO2 YouTube Fe2+ And Kmno4 Equation in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. kmno4 (potassium permanganate) summary of redox reaction. M no− 4 + 8h. Iron + potassium permanganate = hematite + manganese dioxide + potassium oxide. Two moles of iron [fe] and two moles.. Fe2+ And Kmno4 Equation.